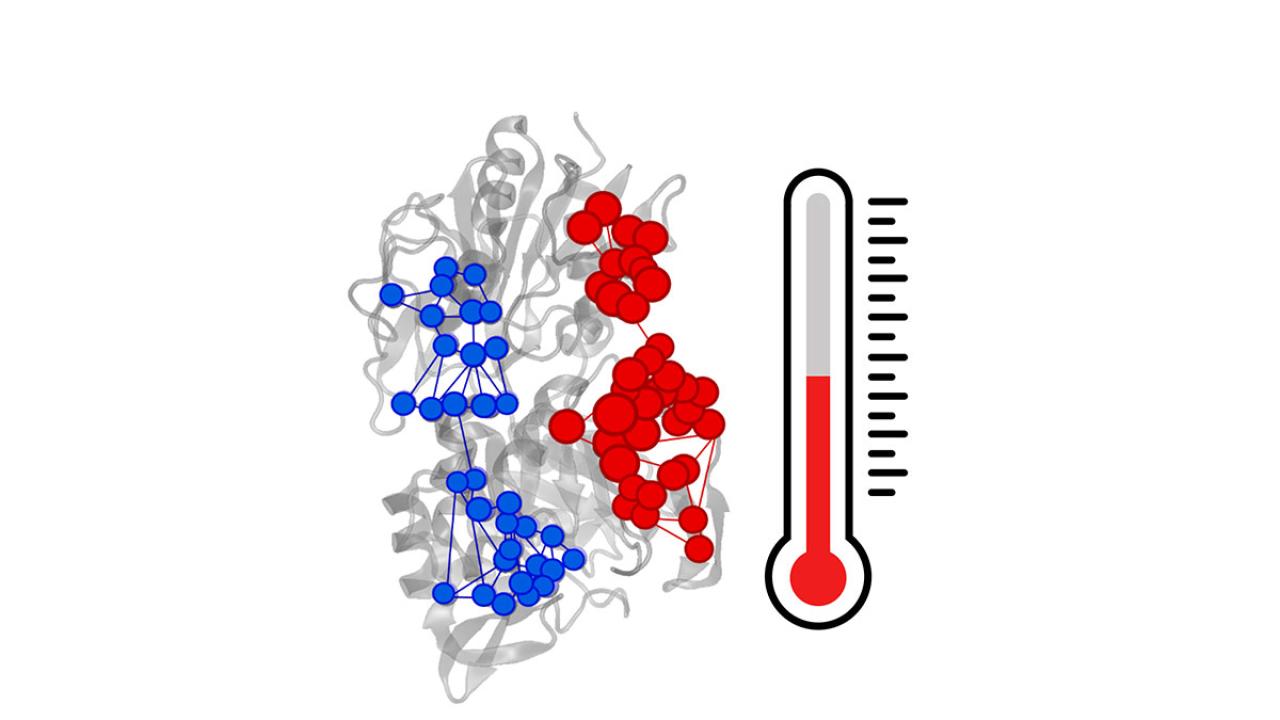
A myriad of fundamental processes in biology rely on the transmission of chemical signals between the spatially separated sites of proteins and enzymes. This phenomenon, called allostery, has intrigued biologists, physicists and chemists alike due to its enormous potential for use in drug discovery applications.
A recent study published in Nature Communications involving researchers at ICTP, Yale University and University of Bologna shows that a temperature increase can trigger a cascade of molecular motions that mimics allosteric signalling.
But how is this relevant to drug discovery? Drugs can be divided in two types: orthosteric and allosteric drugs. In both cases, the therapeutic effect is associated with the drug binding to a protein or an enzyme. This binding then enables or prevents certain biochemical reactions. The main difference between these drug types is their binding position.
“Allosteric drugs are much more selective, since they bind our enzymes far away from where the functional activity takes place,” explains Uriel Morzan, senior research fellow in ICTP's Condensed Matter and Statistical Physics section and co- first author of the study. “Allosteric drugs offer the unique advantage of preventing unexpected side effects and an intrinsic safeguard against overdosage, which shows their tremendous potential for use in both biomedicine and agriculture.”
This interdisciplinary study combines numerical atomistic simulations with graph and information theory approaches to demonstrate that a temperature increase in the enzyme imidazole glycerol phosphate synthase (IGPS) can activate a structural and dynamical response, which surprisingly resembles natural allosteric activation. These results provide fundamental insights into allosteric activation at elevated temperatures.
“We now understand allostery a bit better, and this is exactly what we need if we aim to learn how to control enzymatic activity. IGPS is a potential therapeutic target itself, since it is not present in mammals, but rather in bacteria, some plants and fungi,” says Morzan.
“As physicists, chemists or biologists we are trained to analyse data, biased by our preconceptions of which are the relevant variables that define a problem. Data science approaches offer a uniquely agnostic alternative to learn new physics in those blind spots of our scientific intuition,” adds Morzan. “We discovered this surprising behaviour by looking at an algorithm similar to that employed by Google for ranking webpages. I find this fascinating.”
Link to article:
Turning up the heat mimics allosteric signaling in imidazole-glycerol phosphate synthase. Maschietto, F., Morzan, U.N., Tofoleanu, F. et al. Nat Commun 14, 2239 (2023). https://doi.org/10.1038/s41467-023-37956-1