By simulating how oxygen might behave under the kind of pressure you'd find 300-500 kilometers deep in the Earth's mantle, researchers at ICTP and SISSA have found evidence of a previously unknown form of the element, which they report in the latest issue of the Proceedings of the National Academy of Sciences.
The new phase of oxygen, which arises when oxygen is squeezed 80,000 to 200,000 times more strongly than normal atmospheric pressure, is a solid crystal that appears to be nonmagnetic. Look more closely, though, and each molecule inside the solid is magnetic. It is the dynamic movement and balance of the molecule's magnetic direction that achieves the illusion of a lack of magnetic properties.
This hidden magnetism explains many previously puzzling experimental results that could not be accounted for when it was assumed that the individual molecules were also nonmagnetic. "We've put together a lot of pieces from the literature that were not understood and logically explained them with a hard theoretical background," said ICTP postdoctoral researcher Yanier Crespo, the study's lead author. "That's the beauty of the work."
As a gas, oxygen is as familiar as it is ubiquitous. "Oxygen is one of the most important elements in nature. We wouldn't be here without oxygen," said senior author Erio Tosatti of SISSA. "But it's also rather peculiar from the point of view of its properties."
Oxygen is rare among simple molecules in that each molecule of elemental oxygen, consisting of two oxygen atoms, is attracted to magnetic fields. Its unexpected magnetism stumped chemists until 1929, when John Lennard-Jones demonstrated that quantum mechanics could explain magnetic oxygen, something that standard chemical theory based on classical mechanics had been unable to do.
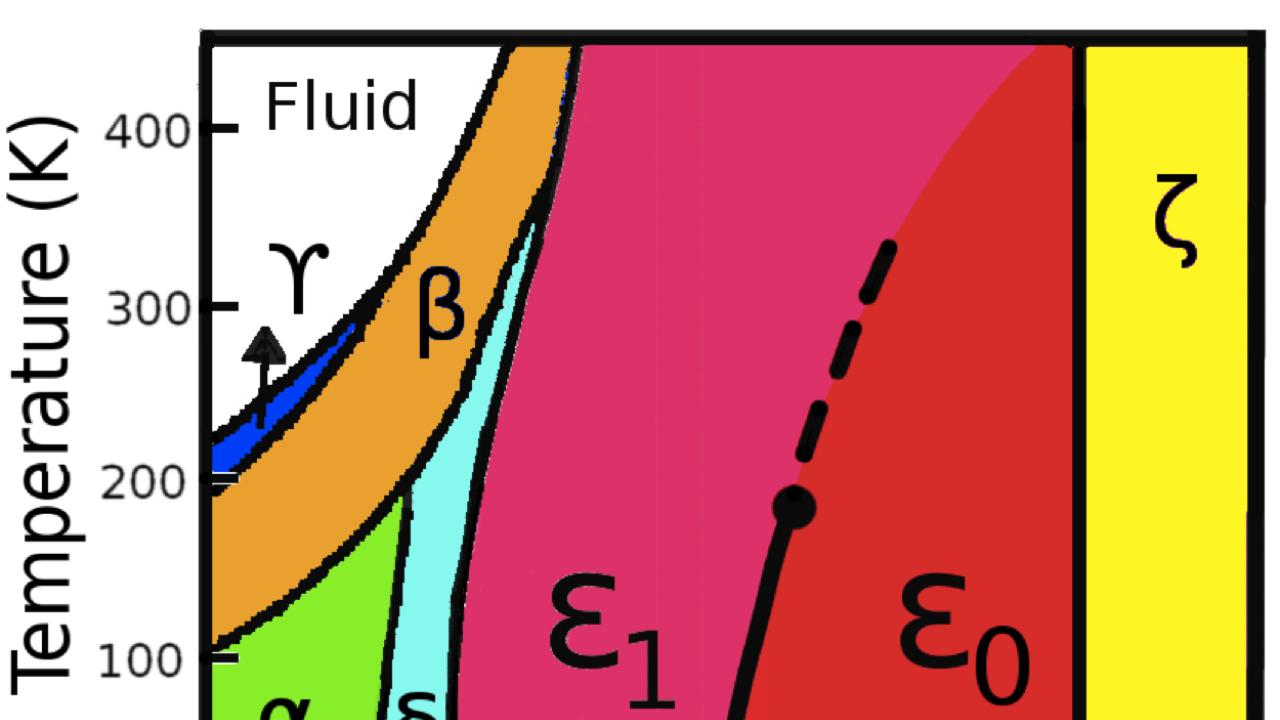
Magnetism is not the only mystery about oxygen, however. "There are lots of surprises in the phase diagram of oxygen," Tosatti said, referring to a graph which shows what state (for example, solid, liquid, or gas) a particular material will be in at a given temperature and pressure. One such surprise is that squeezing oxygen at 1 million times normal atmospheric pressure turns it into a metallic semiconductor.
At a slightly lower pressure, between 80,000 and 960,000 times atmospheric pressure, oxygen enters the enigmatic "epsilon phase," the largest swath of its phase diagram, which Tosatti called "an unexplored Siberia." Experiments on the lower-pressure region of the epsilon phase had so far yielded conflicting results. "We were in a puzzle, where some experiments suggest that magnetism is present and others show that magnetism is not there," Crespo said.
Part of the puzzle was explained eight years ago, when research groups in France and Japan simultaneously used X-rays to reveal the structure of the epsilon phase: the molecules arranged themselves in unusual groups of four, or quartets, that were not magnetic.
Still, the understanding of the epsilon phase was incomplete. "This picture explains everything in the high pressure range, but is bad at lower pressure," Tosatti said. So he and the team of researchers at ICTP and SISSA decided to examine the epsilon phase in finer detail using theoretical techniques developed over the last 20 years to simulate the behavior, especially the quantum effects, of matter at high pressure.
"These calculations are remarkable because they become more accurate the higher the pressure," Tosatti said, something which is not true of experiments. The theory has a further advantage over experiments: "In the theory, nothing explodes."
When the ICTP scientists simulated the oxygen at the lower pressures of the epsilon phase, they found that the molecules were most likely to retain their ability to interact with a magnetic field. However, in order to do so, they must constantly move their magnetic tendencies in such a way that the average cancels out. At any given moment in each quartet, two molecular magnets point up and two point down.
Motivated by these results, the scientists went on to test the implications of this arrangement for experimentally tested properties of the epsilon phase. The more they looked in the literature, the more the scientists found evidence of curious phenomena in the lower range of pressures that no one had been able to explain. In most cases, the new arrangement was enough to account for oxygen's odd behavior in the lower part of the epsilon phase, leading the researchers to call for the splitting of the epsilon phase into two separate parts: the previously known nonmagnetic phase between 200,000 and 960,000 times atmospheric pressure, and the new "spin liquid" phase between 80,000 and 200,000 times atmospheric pressure.
Unlike the traditional sense of a liquid, where molecules move around more freely than in a solid because of a higher temperature, in a spin liquid it is the spins, or directions in which each molecule's magnetic attraction is pointing, that move. Each molecule flips its spin up and down, and the other three members of its group respond in a perpetual dance to keep the overall magnetism of the material at zero.
Spin liquids like this are very rare, and Crespo believes that the discovery will open the door to even more interesting science. "There is a lot of physics there that hasn't been studied yet," he said.
Already, the scientists have been in contact with experimental colleagues around the world to test out the theoretical predictions more directly. "It will not take too long before they can say whether we are right or wrong," Tosatti said.